22 October 2021
American drug-maker Pfizer says a smaller dose of its COVID-19 vaccine appears safe and 91 percent effective at preventing infections in 5- to 11-year-old children.
The company released a study with the findings on Friday. The United States is considering whether to vaccinate children of that age. The usual dose of the vaccine, made by Pfizer and its German partner BioNTech, has already been approved for people 12 and older.
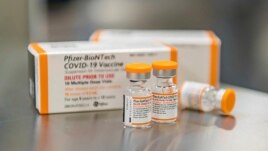
This October 2021 photo provided by Pfizer shows kid-size doses of its COVID-19 vaccine in Puurs, Belgium. The drug-maker says the vaccine appears safe and nearly 91% effective at preventing symptomatic infections in 5- to 11-year-olds.
The U.S. Food and Drug Administration (FDA) is studying the information. If the agency approves the smaller dose for children, the Centers for Disease Control and Prevention will decide who should receive them. The injections could be available in early November.
More than 25,000 doctors and healthcare providers already have requested the vaccines. The administration of President Joe Biden has also paid for enough child-size doses for the nation's 28 million 5- to 11-year-olds.
The Pfizer study released Friday followed 2,268 children. They received two shots three weeks apart of either a placebo -- an inactive substance -- or a smaller dose of the vaccine. The smaller dose was one-third the amount given to people 12 and older.
Researchers said the vaccine was nearly 91 percent effective. They found 16 COVID-19 cases among children receiving the placebo and three cases in children who received the actual shots.
There were no severe illnesses reported among any of the children. But the vaccinated children had weaker symptoms than the unvaccinated ones.
In addition, young children given the smaller doses developed coronavirus-fighting antibody levels as strong as those found in teens and young adults who got the normal shots.
Hospitalizations of mostly unvaccinated children reached record high levels last month in the U.S.
The CDC reported earlier this week that Pfizer vaccinations were 93 percent effective at preventing hospitalizations among 12- to 18-year-olds. New infections are being blamed on the highly infectious Delta variant spreading across the country.
Pfizer's study of younger children found the shots to be safe, with similar or fewer side effects such as pain at the place of the injection and a temporary higher body temperature.
Children have a lower risk of severe illness or death than older people. The American Academy of Pediatrics said nearly 6.2 million American children have been infected with the coronavirus. More than 1.1 million have been infected in the last six weeks.
Drug-maker Moderna also is studying its COVID-19 shot in younger children. In addition to that, Pfizer and Moderna are studying the shots in children as young as six months old. Results are expected later in the year.
I'm Ashley Thompson.
Lauran Neergaard and Mathew Perrone reported this story for the Associated Press. Hai Do adapted it for VOA Learning English. Mario Ritter, Jr. was the editor.
____________________________________________________
Words in This Story
dose – n. the amount of a medicine, drug or vitamin that is taken at one time
symptom –n. a change in the body or mind that shows that a disease is present
We want to hear from you. Write to us in the Comments section, and visit 51VOA.COM.